KBM products
KLS Martin® is pleased to offer the gold standard in regenerative products through a partnership with Xtant Medical, a fully accredited orthobiologic manufacturer and American Association of Tissue Banks member.
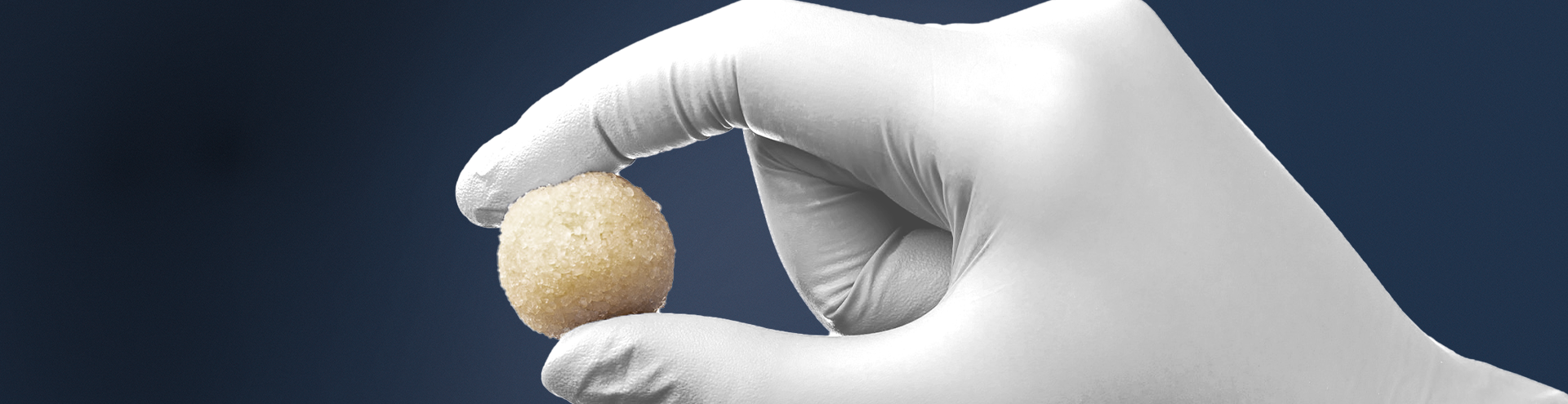
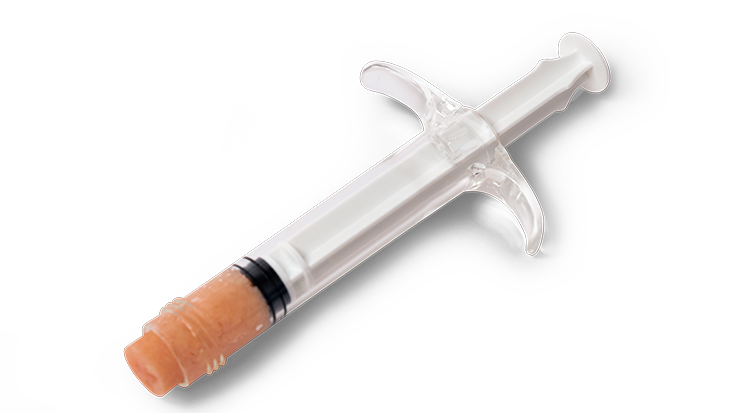
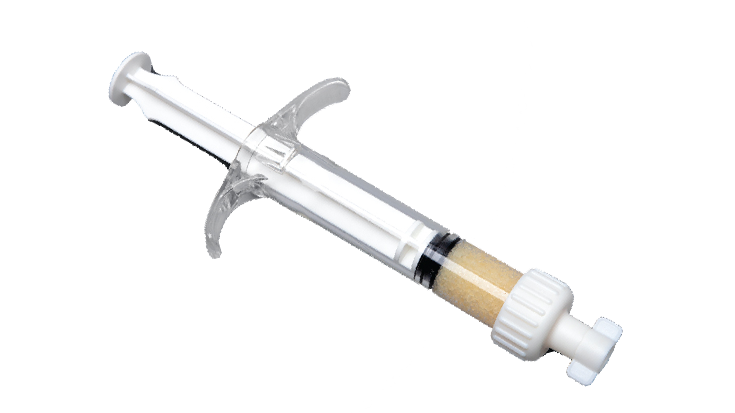
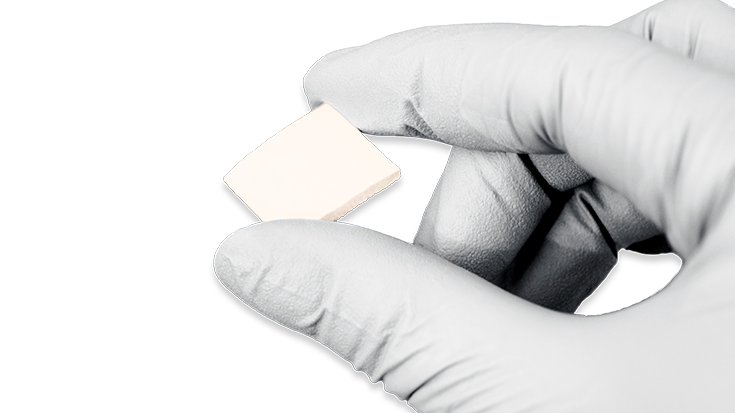
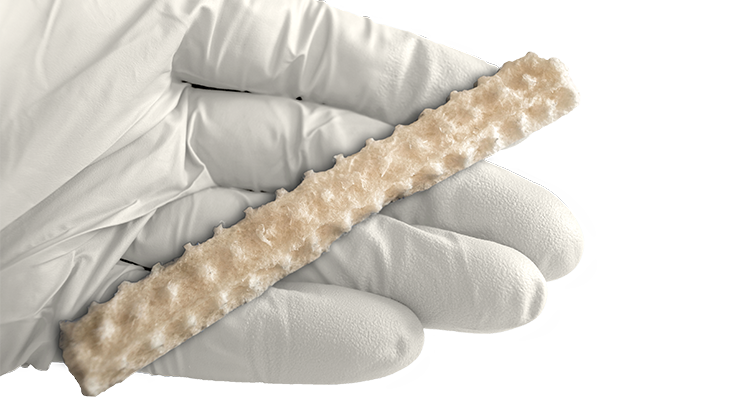
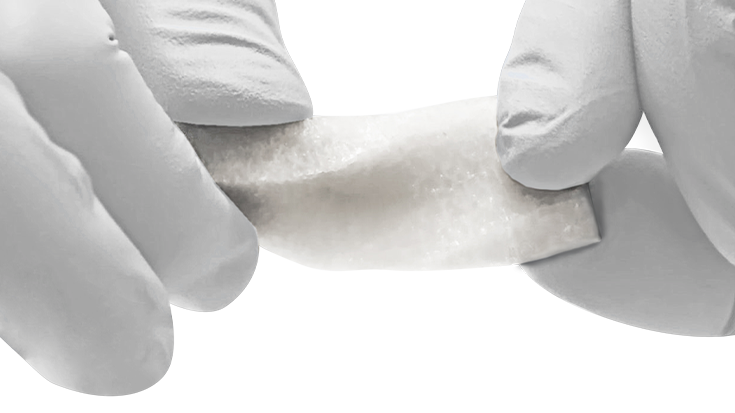
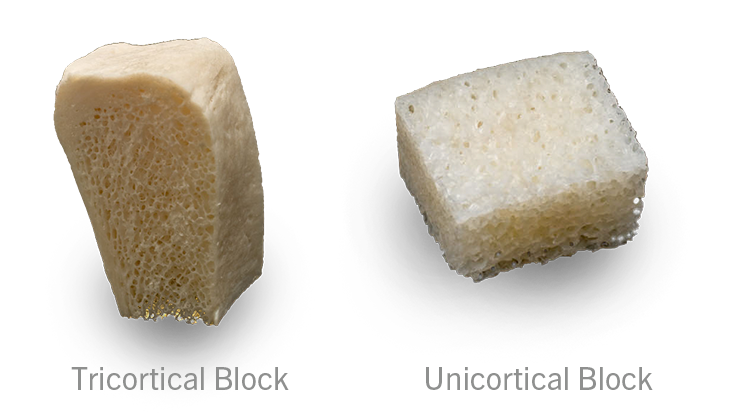
KBM (KLS Bone Matrix) products not only provide an osteoconductive scaffold but have demonstrated osteoinductive potential when tested in vivo. Therefore, safety is of the utmost importance, and we provide each implant at device-level sterility. This level is the highest in the industry, providing a sterility assurance level (SAL) of 10-6.
All KBM products (except for KBM OsteoSelect Putty) are regulated under Section 361 of the Public Health and Service Act as allograft tissues and have the intention of homologous use.
Learn more about our solutions here.