"The unique device identification (UDI) is a unique numeric or alphanumeric code related to a medical device. It allows for a clear and unambiguous identification of specific devices on the market and facilitates their traceability. The UDI comprises the following components
These components provide access to useful information about the device. The specificity of the UDI
The UDI will be an addition to, not a substitute for, the existing labelling requirements for medical devices." (European Commission 2023)
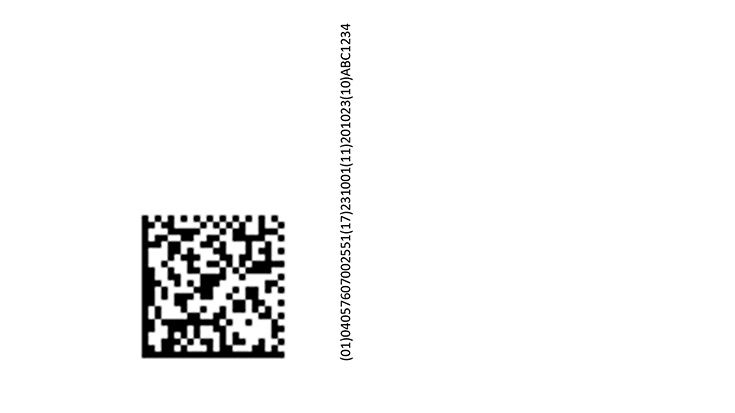
(01)04057607002551 UDI-DI GTIN, per packaging level
(17)YYMMDD UDI-PI Expiry date, if required
(11)YYMMDD UDI-PI Date of manufacture
(10)ABC1234 UDI-PI Batch number/lot number
or alternatively / and
(21)A283723 UDI-PI serial number
UDI-DI/GTIN = Distinctive coding with GS1 standard based on ISO/IEC 16022 and with ECC200 error correction. The image shows a sample GS1 DataMatrix code (this is not to scale).
Permissible magnification factors (X-factor) are between 0.254 mm and 0.995 mm. The print quality must be at least Grading C (1.5/08/660).
1European Commission 2023: Unique Device Identifier (UDI), European Commission, [online] https://health.ec.europa.eu/medical-devices-topics-interest/unique-device-identifier-udi_de [retrieved on 19.06.2023].