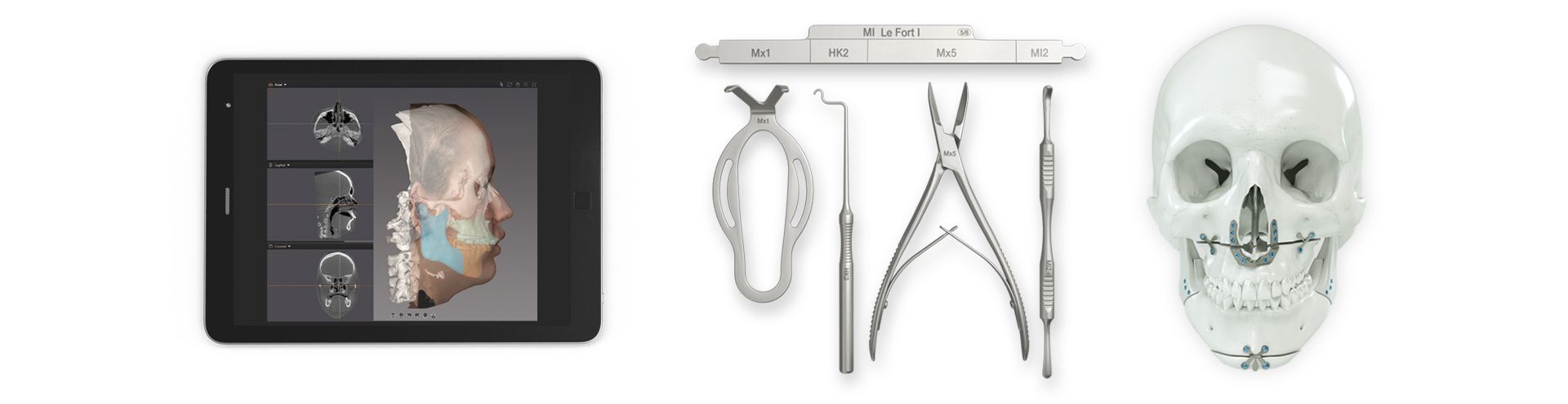
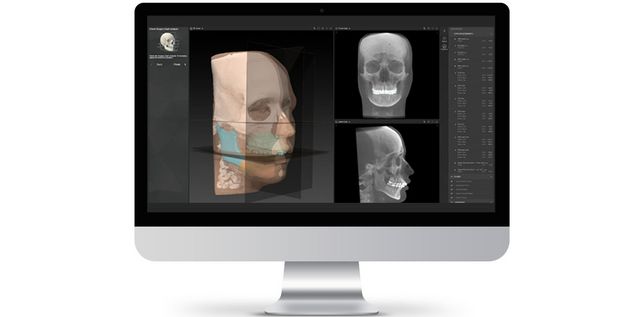
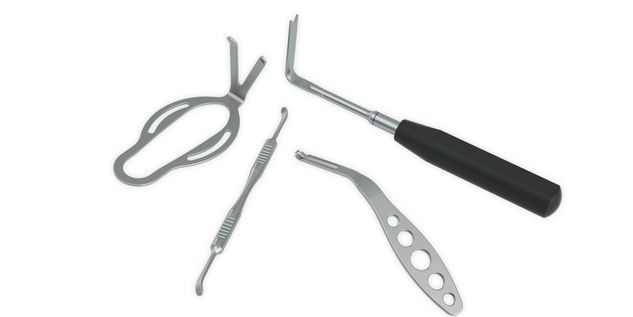
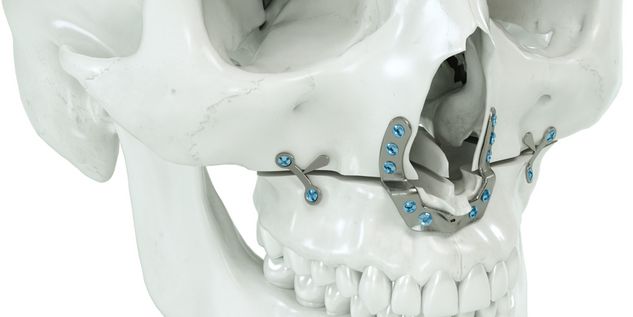
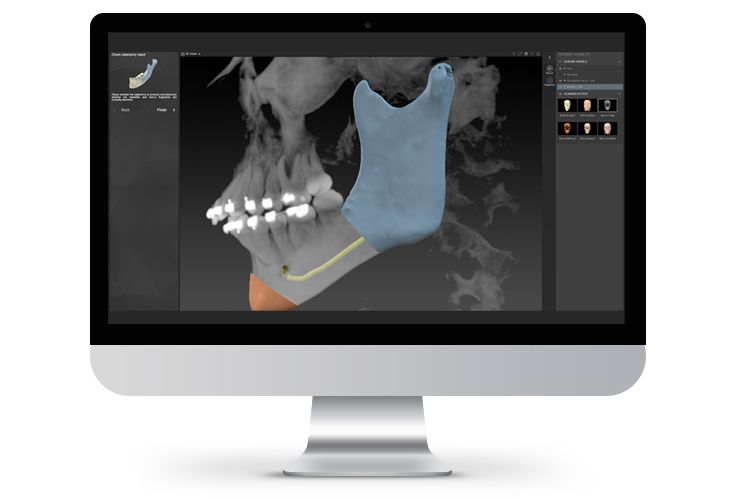
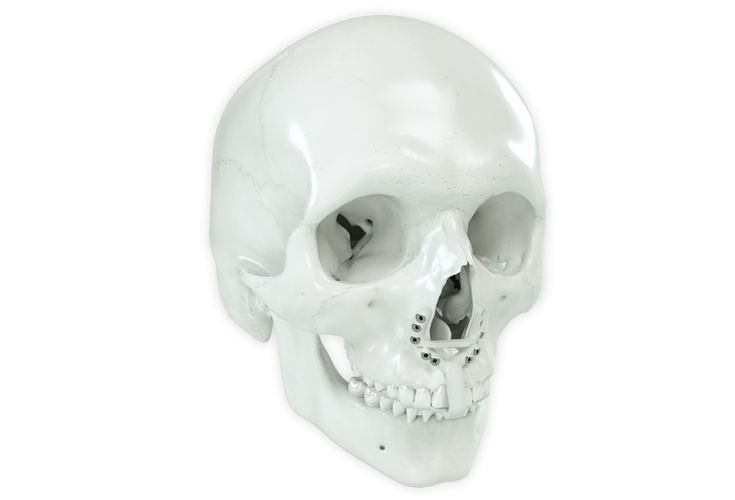
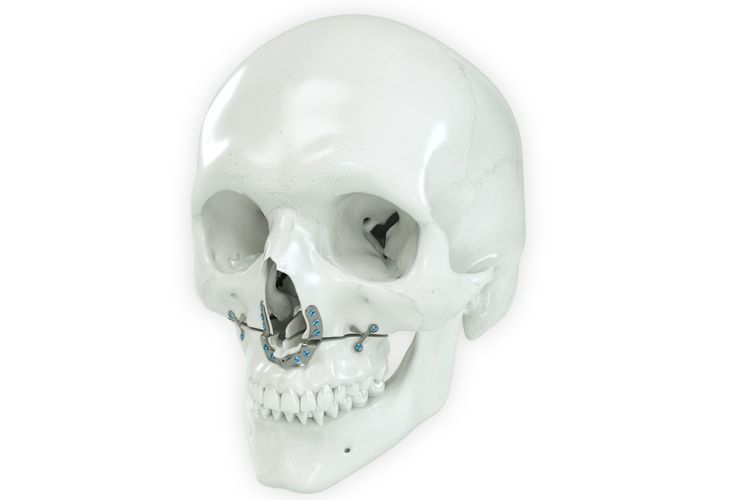
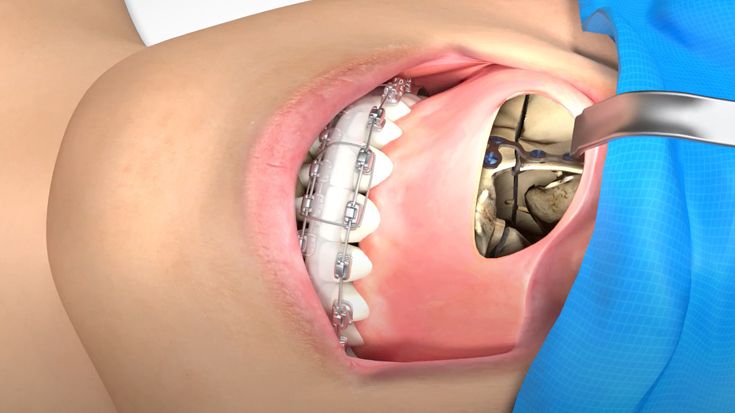
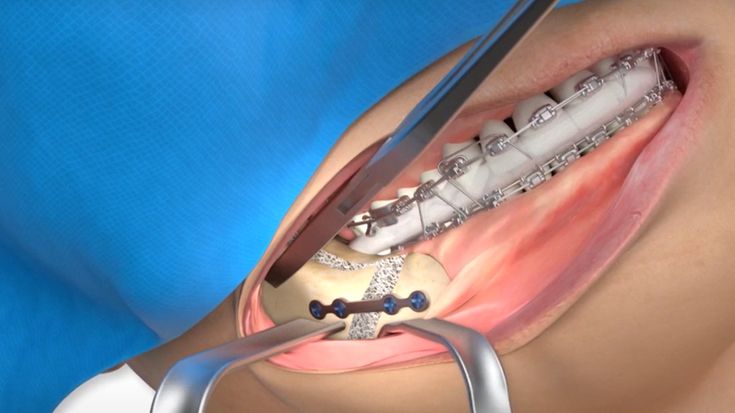
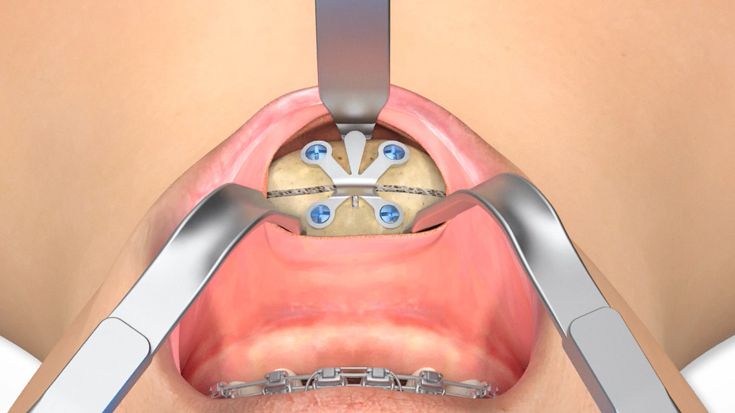
According to the regulation (EU) 2017/745, a custom-made product is a device specifically made in accordance with a written prescription of any person authorized by national law by virtue of that person's professional qualifications which gives, under that person's responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
For this reason a separate inquiry is necessary in every IPS® case.
The written prescription is a release for the technical offer (design of the products). This is to be submitted in writing by the user at the same time as the required order for the economic offer if he/she agrees with the desired case planning.
Shipment is not permissible as a matter of principle without this mandatory regulatory document.