Individual Patient Solutions
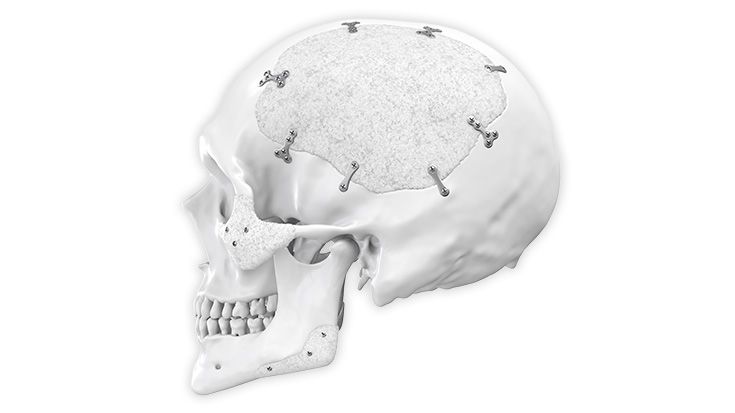
IPS Implants®
The right "customized" choice for every patient
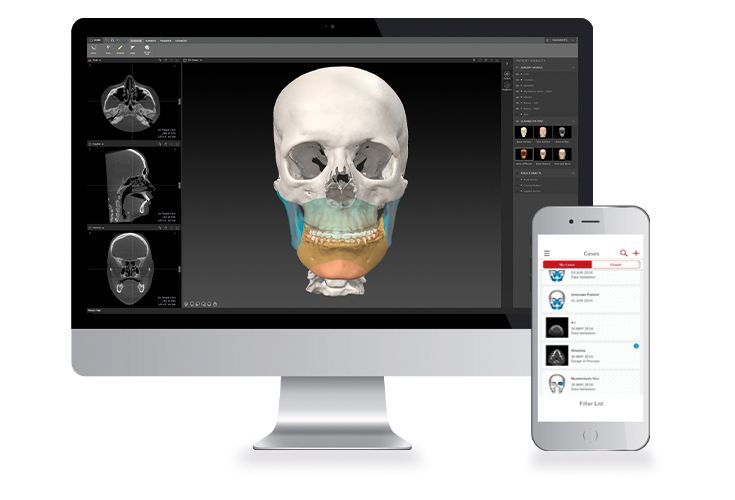
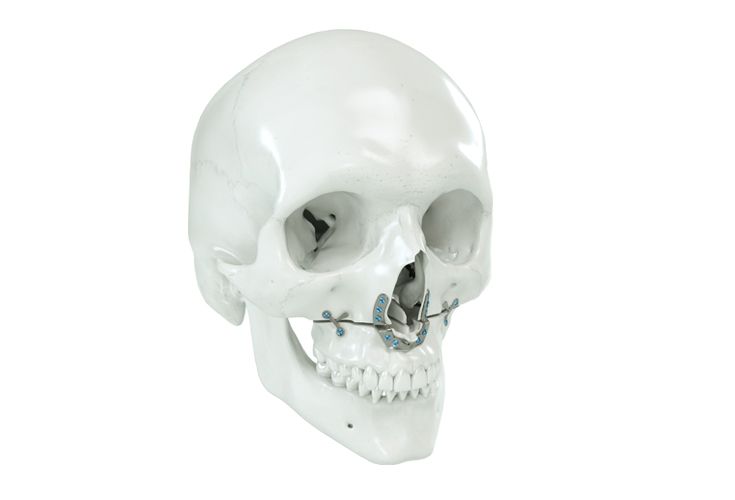
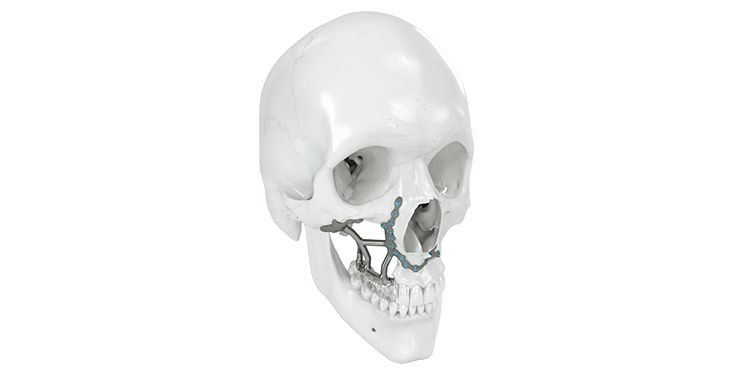
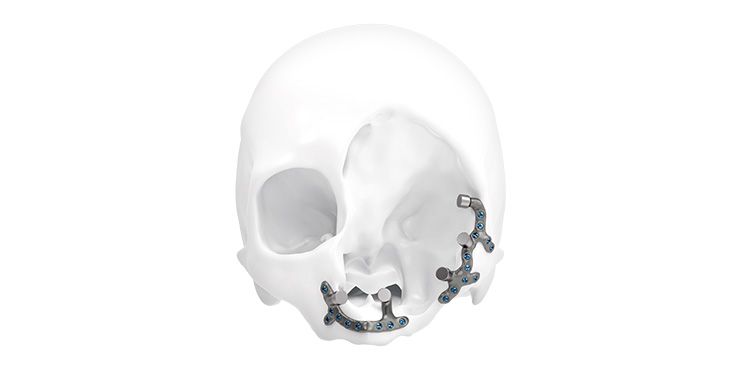
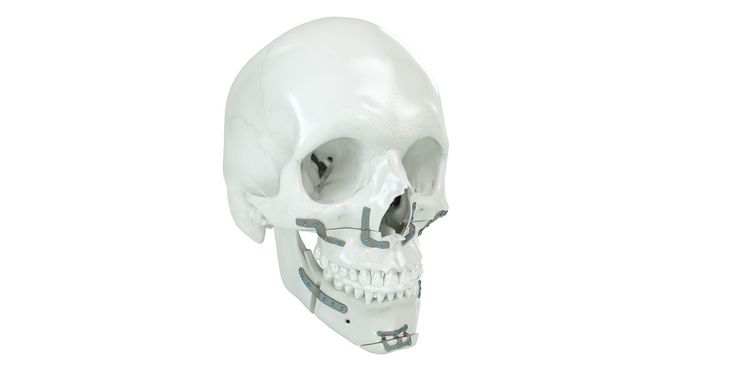
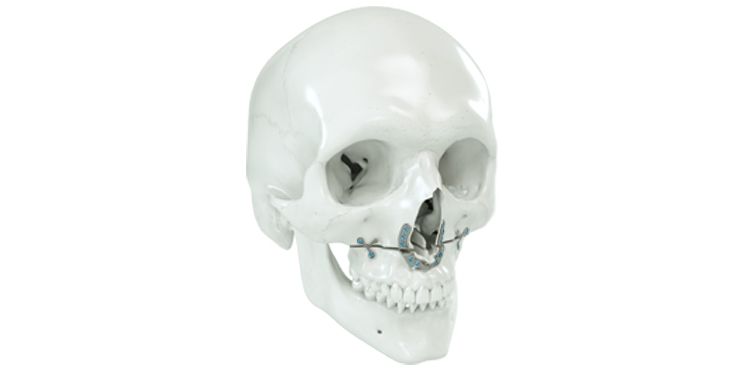
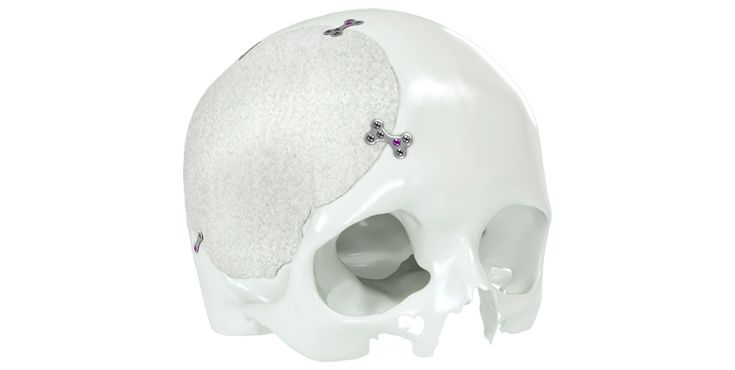
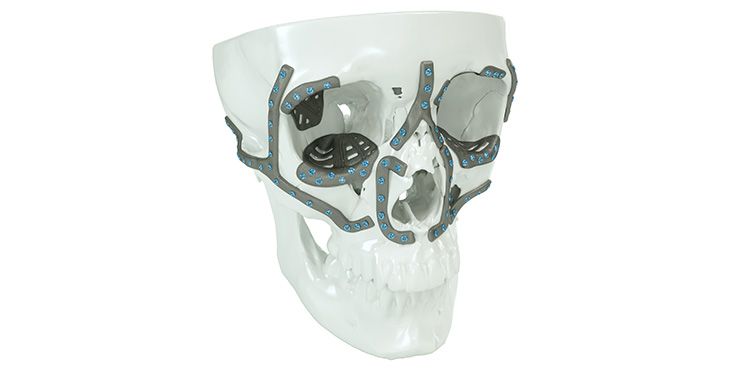
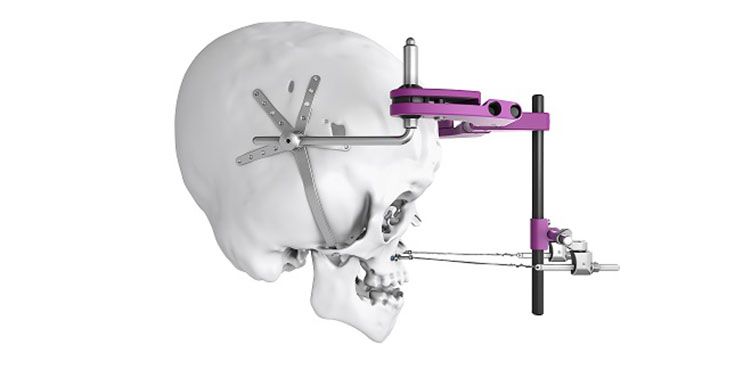
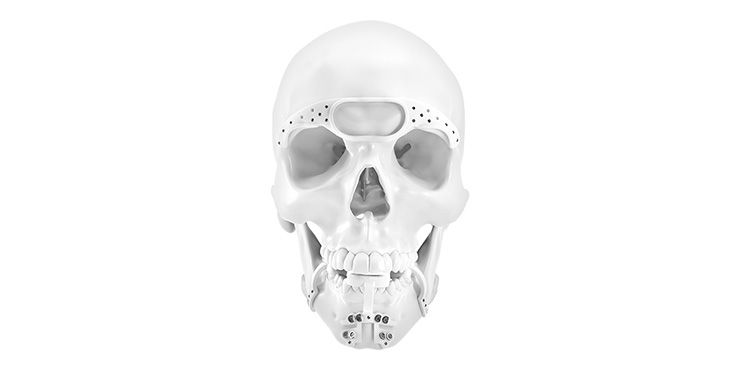
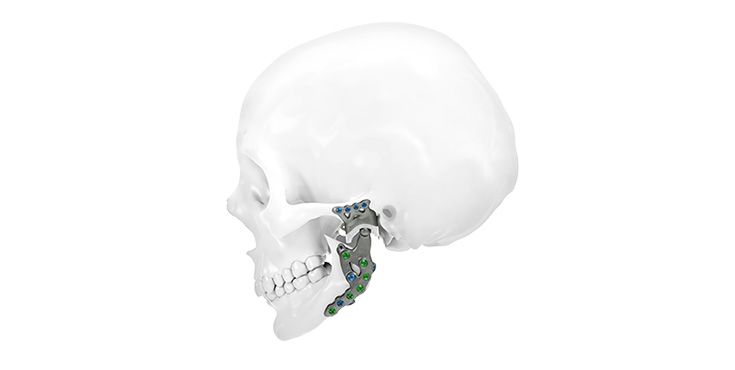
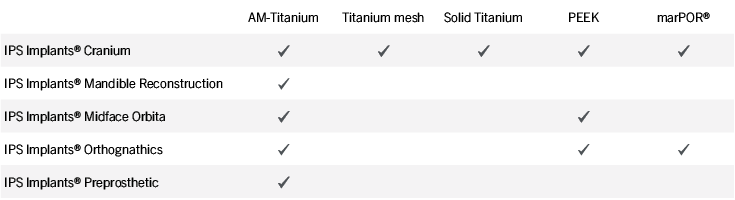
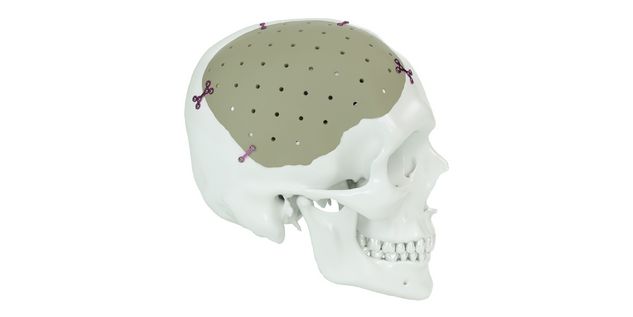
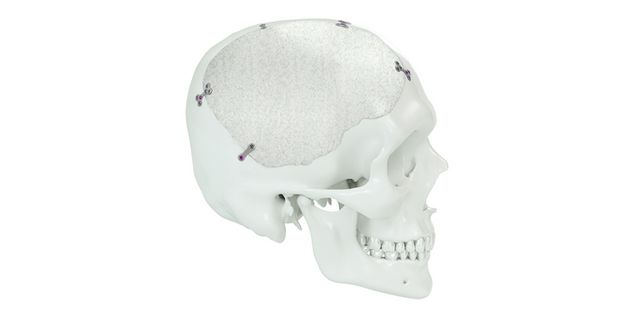
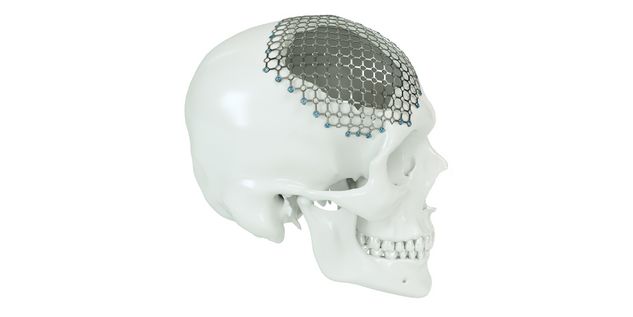
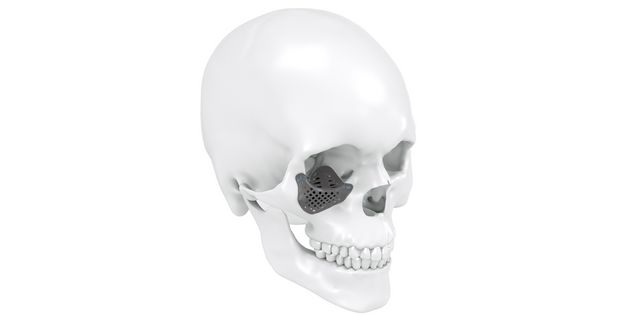
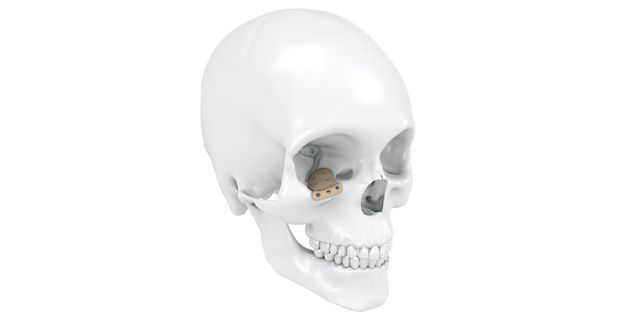
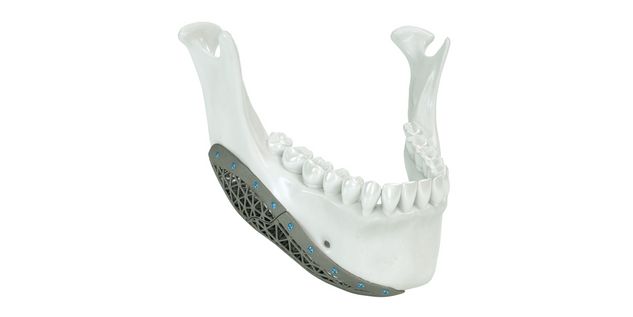
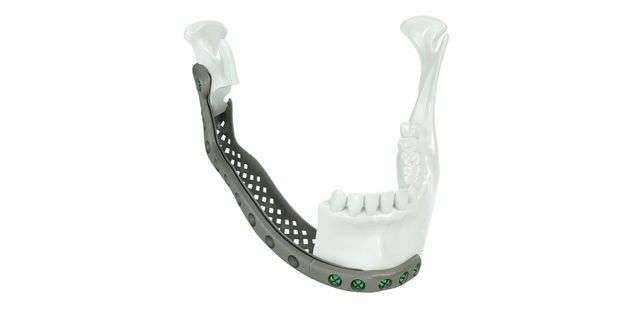
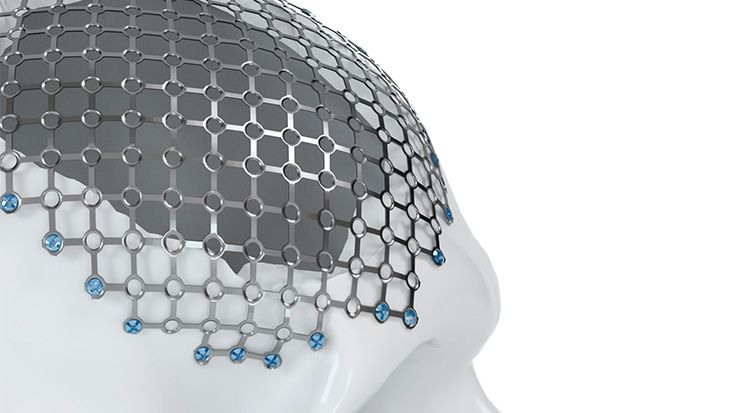
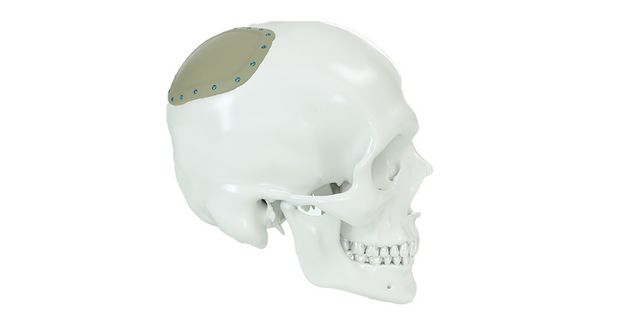
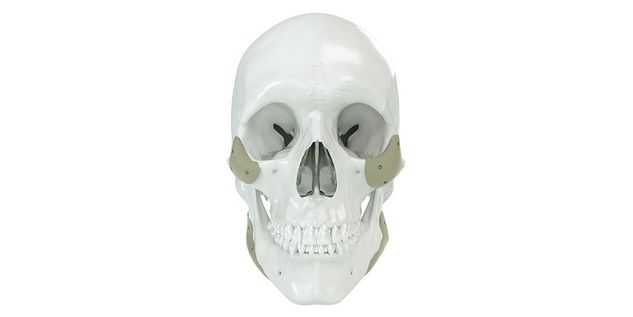
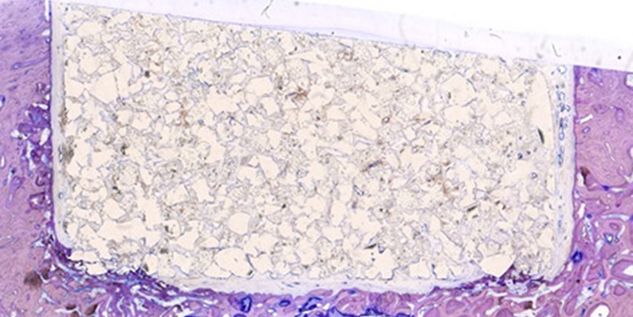
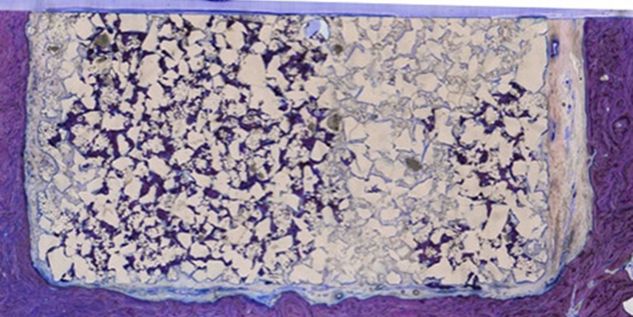
Patient-specific implants, planning aids, and anatomical models are made from various materials using state-of-the-art fabrication technologies. Thanks to computer-based planning and functionalized patient-specific implants, preoperative planning can be implemented in surgery with unprecedented precision.
Based on the potential planning in our IPS CaseDesigner® software and subsequent case treatment and communication in the IPS Gate®, you will then receive an IPS® implant fabricated to your wishes. As a result, the IPS® product range offers a versatile service in terms of patient-specific implants.